Permeation is the movement of gas and vapor through a barrier such as the wall of a bottle. It is driven by the permeant concentration gradient that always happens from the permeant’s high concentration side to the low concentration side.

Fig 1. Permeation a Natural Process
For example, imagine there is a bottle of carbonated soft drink (Fig 1). The CO2 within the product is about 4 atm (when it is freshly filled) while the CO2 concentration in environment is less than 0.5% in the air. Therefore, the CO2 will permeate from inside bottle towards the outside. Similarly, the oxygen from room air will permeates from the outside of the bottle to inside. Although permeation process is invisible, it can be detected by the CO2 concentration loss over time. Or, simply you can taste the soft drink. The flat taste of the liquid indicates the loss of CO2 which lead to the loss of its premier quality or shelf life.
 Fig 2. Solution-Diffusion Mechanism
Fig 2. Solution-Diffusion Mechanism
The permeation mechanism has three steps (Fig 2):
- Permeant molecules absorb into the surface (high concentration side)
- Permeant molecules move or diffuse through the barrier material
- Permeant molecules desorb out of the other side (low concentration side)
Therefore, permeation is related to both solubility (S) and diffusivity (D) with the following math equation:
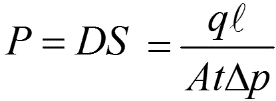
P = permeability coefficient
D = diffusion coefficient
S = solubility coefficient
q = quantity of permeant transferred by a unit of area, A, in a time t, is the thickness of material and Δp is the partial pressure difference.
In practical applications, the transmission rate (TR), is the most common way to report the “flux” of gas moving through a polymer. It also makes the most sense, as many polymers are multi-layered or coated. The “net flux” of oxygen, water vapor, carbon dioxide…etc. is what’s important to a product’s shelf-life.
Permeation and Transmission Rate have the following units:


Above equations demonstrate that the permeation rate is the thickness and driving force normalized transmission rate.
Related Links: